Abstract
Background: Zanubrutinib (zanu) is a selective next-generation Bruton tyrosine kinase (BTK) inhibitor designed to have high specificity for BTK and minimize off-target effects (Guo, J Med Chem 2019;62:7923-40). In a phase 1/2 study, zanu demonstrated complete and sustained BTK occupancy in both peripheral blood mononuclear cells and lymph nodes and was associated with durable clinical responses in patients (pts) with CLL/SLL (Tam, Blood 2019;134:851-9). Here, we present interim results for the phase 3 SEQUOIA (BGB-3111-304; NCT03336333) trial, which evaluated the efficacy and safety of zanu vs BR in TN pts with CLL/SLL.
Methods: SEQUOIA is an open-label, global phase 3 study that randomized TN pts with CLL/SLL without del(17p) to receive zanu 160 mg twice daily until progressive disease or unacceptable toxicity, or bendamustine 90 mg/m 2 on day 1 and 2 and rituximab 375 mg/m 2 in cycle 1, 500 mg/m 2 in cycles 2-6 for 6 × 28-day cycles. Adult pts with CLL/SLL who met International Workshop on CLL (iwCLL) criteria for treatment (Hallek, Blood 2008;111:5446-56) were eligible if they were either ≥65 y or unsuitable for treatment with fludarabine, cyclophosphamide and rituximab. Central verification of del(17p) status by fluorescence in situ hybridization was required. Pts were stratified by age (<65 y vs ≥65 y), Binet Stage (C vs A/B), IGHV mutational status, and geographic region. The primary endpoint was independent review committee (IRC)-assessed progression-free survival (PFS) for zanu vs BR. Secondary endpoints included PFS by investigator assessment (INV), overall response rate (ORR; by IRC and INV), overall survival (OS), and safety. Responses for CLL and SLL were assessed per modified iwCLL criteria (Hallek, Blood 2008;111:5446-56; J Clin Oncol 2012;30:2820-2) and Lugano criteria (Cheson, J Clin Oncol 2014;32:3059-68), respectively. Adverse events (AEs) were recorded until disease progression to support safety evaluation over an equivalent time period.
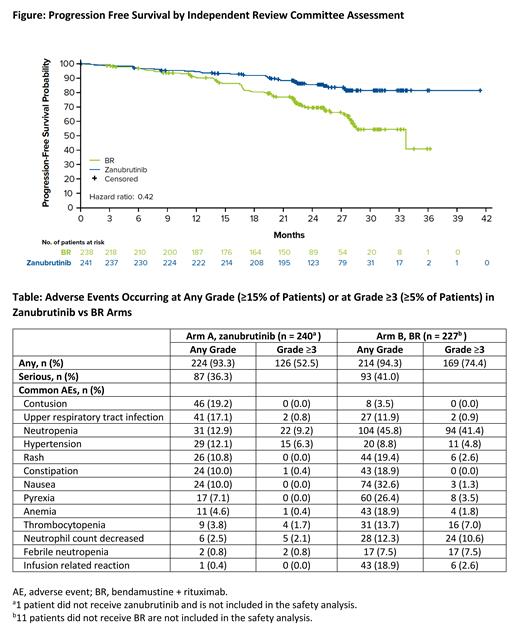
Results: From 31 Oct 2017-22 Jul 2019, 479 pts without del(17p) were randomized to zanu (n=241) and BR (n=238). Treatment groups were well balanced for demographic and disease characteristics (zanu vs BR): median age, 70.0 y vs 70.0 y; unmutated IGHV, 53.4% (125/234) vs 52.4% (121/231); and del(11q), 17.8% vs 19.3%. At median follow-up (26.2 mo), PFS by IRC was significantly prolonged with zanu vs BR (HR 0.42, 95% CI 0.28-0.63, 1-sided and 2-sided P<0.0001; Figure); similar results were observed by INV (HR 0.42, 95% CI 0.27-0.66, 1-sided P<0.0001, 2-sided P=0.0001). Treatment benefit for zanu was observed across subgroups for age, Binet stage, bulky disease, and del(11q) status. Treatment benefit was also observed for pts with unmutated IGHV (HR 0.24, 1-sided and 2-sided P<0.0001), but not for mutated IGHV (HR 0.67, 1-sided P=0.0929). Estimated 24-mo PFS (IRC) for zanu vs BR was 85.5% (95% CI 80.1%- 89.6%) vs 69.5% (95% CI 62.4%-75.5%). ORR by IRC for zanu vs BR was 94.6% (95% CI 91.0%-97.1%) vs 85.3% (95% CI 80.1%-89.5%). Complete response rate was 6.6% with zanu and 15.1% with BR. ORR by INV for zanu vs BR was 97.5% (95% CI 94.7%-99.1%) vs 88.7% (95% CI 83.9%-92.4%) Estimated 24-mo OS for zanu vs BR was 94.3% (95% CI 90.4%-96.7%) and 94.6% (95% CI 90.6%-96.9%).
The most common AEs are shown in the Table. AEs of interest occurring during the full reporting period (pooled terms, zanu vs BR) included atrial fibrillation (any grade [gr]: 3.3% vs 2.6%), bleeding (any gr/gr≥3: 45.0%/3.8% vs 11.0%/1.8%), hypertension (any gr: 14.2% vs 10.6%), infection (any gr/gr≥3: 62.1%/16.3% vs 55.9%/18.9%), and neutropenia (any gr/gr≥3: 15.8%/11.7% vs 56.8%/51.1%). Treatment discontinuation due to AEs occurred in 20 pts (8.3%) receiving zanu vs 31 pts (13.7%) receiving BR; 85.5% of pts receiving zanu remain on treatment. AEs leading to death occurred in 11 pts (4.6%) receiving zanu vs 12 pts (5.3%) receiving BR. No sudden deaths were reported.
Conclusions: In this global registrational trial, zanu demonstrated statistically significant improvement in PFS compared to BR as assessed by IRC. Superiority was also observed in PFS by INV as well as ORR by both IRC and INV. Zanu was generally well tolerated, with low rates of atrial fibrillation consistent with those observed in the phase 3 ASPEN (Tam, Blood 2020;136:2038-2050) and ALPINE studies (Hillmen, EHA 2021 #LB1900). These data support the potential utility of zanu in the frontline management of pts with TN CLL/SLL.
Tam: AbbVie: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Pharmacyclics: Honoraria; Novartis: Honoraria; Loxo: Consultancy; Roche: Consultancy, Honoraria. Giannopoulos: Polish Myeloma Consortium, Next Generation Hematology Association: Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Honoraria; Pfizer: Honoraria; Teva: Honoraria; TG Therapeutics: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Astra-Zeneca: Consultancy, Honoraria, Research Funding; Bei-Gene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Sanofi-Genzyme: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Gilead: Honoraria, Research Funding. Jurczak: Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Bayer: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celtrion: Research Funding; Celgene: Research Funding; Debbiopharm: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Loxo Oncology: Membership on an entity's Board of Directors or advisory committees; Sandoz: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Epizyme: Research Funding; Incyte: Research Funding; Merck: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding. Šimkovič: Janssen, Gilead, Roche, AstraZeneca, and AbbVie: Other: consultancy fees, advisory board participation fees, travel grants, and honoraria; University Hospital Hradec Kralove: Current Employment; AbbVie: Consultancy, Current equity holder in publicly-traded company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Merck: Current equity holder in publicly-traded company; Eli Lilly: Current equity holder in publicly-traded company; J&J: Current equity holder in publicly-traded company; Gilead: Other: Travel, Accommodations, Expenses. Shadman: Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune , Mustang Bio and Atara Biotherapeutics: Consultancy; Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, Abbvie, TG Therapeutics, Beigene, AstraZeneca, Sunesis, Atara Biotherapeutics, GenMab: Research Funding. Österborg: BeiGene: Research Funding; Gilead: Research Funding. Laurenti: Janssen: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Gilead: Honoraria; Roche: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; BeiGene: Honoraria. Walker: BeiGene: Consultancy; Acerta: Consultancy. Opat: Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Sandoz: Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Monash Health: Current Employment. Chan: AbbVie: Membership on an entity's Board of Directors or advisory committees; Eusa: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Membership on an entity's Board of Directors or advisory committees; Amgen: Other: Travel, Accommodations, Expenses; Celgene: Other: Travel, Accommodations, Expenses; Roche: Speakers Bureau. Ciepluch: Copernicus Wojewodzkie Centrum Onkologii: Current Employment. Greil: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Sandoz: Honoraria, Research Funding; Amgen: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Daiichi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses, Research Funding. Trněný: AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Portola: Honoraria, Membership on an entity's Board of Directors or advisory committees; MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; 1st Faculty of Medicine, Charles University, General Hospital in Prague: Current Employment; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Celgene: Consultancy; Amgen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Gilead Sciences: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; AstraZeneca: Honoraria. Brander: Verastem: Consultancy; ArQule: Research Funding; Genentech: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; Juno Therapeutics/Celgene/Bristol Myers Squibb: Research Funding; MEI Pharma: Research Funding; Novartis: Research Funding; LOXO: Research Funding; Ascentage: Research Funding; AstraZeneca: Research Funding; BeiGene: Research Funding; DTRM: Research Funding; AbbVie: Consultancy, Other: informCLL registry steering committee, Research Funding; Pfizer: Consultancy, Other: Biosimilars outcomes research panel; NCCN: Other: panel member; ArQule/Merck: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding. Flinn: Loxo: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Juno Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Nurix Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; IGM Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Forma Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Forty Seven: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; MorphoSys: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Roche: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Janssen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Novartis: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Takeda: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Great Point Partners: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Genentech: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; AstraZeneca: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; AbbVie: Consultancy, Other: All Consultancy and Research Funding payments made to Sarah Cannon Research Institute, Research Funding; Gilead Sciences: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Celgene: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Curis: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Infinity Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Karyopharm Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Kite, a Gilead Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Incyte: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Merck: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; BeiGene: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Calithera Biosciences: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Seagen: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; TG Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Iksuda Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Constellation Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; ArQule: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Unum Therapeutics: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Agios: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Verastem: Consultancy, Other: All consultancy and research funding payments made to Sarah Cannon Research Institute, Research Funding; Acerta Pharma: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Trillium Therapeutics: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Teva: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Rhizen Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Portola Pharmaceuticals: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Pfizer: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Triphase Research & Development Corp.: Other: All research funding payments made to Sarah Cannon Research Institute, Research Funding; Century Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Hutchison MediPharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Vincerx Pharma: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Sarah Cannon Research Institute: Current Employment; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Yingli Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Seagen: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Servier Pharmaceuticals: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute; Unum Therapeutics: Consultancy, Other: All consultancy payments made to Sarah Cannon Research Institute, Research Funding; Johnson & Johnson: Current holder of individual stocks in a privately-held company; Seattle Genetics: Research Funding. Verner: Janssen-Cilag Pty Ltd: Research Funding. Brown: Janssen: Consultancy; MEI Pharma: Consultancy; Rigel: Consultancy; Bristol-Myers Squib/Juno/Celegene: Consultancy; Novartis: Consultancy; Invectys: Other: Data Safety Monitoring Committee Service; TG Therapeutics: Research Funding; Abbvie: Consultancy; Acerta/Astra-Zeneca: Consultancy; Beigene: Consultancy; Catapult: Consultancy; Loxo/Lilly: Research Funding; Sun: Research Funding; Nextcea: Consultancy; Gilead: Research Funding; SecuraBio: Research Funding; Eli Lilly and Company: Consultancy; Genentech/Roche: Consultancy; Pfizer: Consultancy; Morphosys AG: Consultancy. Kahl: AbbVie, Adaptive, ADCT, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Genentech, Incyte, Janssen, Karyopharm, Kite, MEI, Pharmacyclics, Roche, TG Therapeutics, and Teva: Consultancy; AbbVie, Acerta, ADCT, AstraZeneca, BeiGene, Genentech: Research Funding. Ghia: Sunesis: Research Funding; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Research Funding; Celgene/Juno/BMS: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; ArQule/MSD: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Acerta/AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Tian: BeiGene: Current Employment; AbbVie: Ended employment in the past 24 months. Marimpietri: BeiGene USA: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Paik: BeiGene USA, Inc.: Current Employment, Current equity holder in publicly-traded company. Cohen: BeiGene: Current Employment, Current equity holder in publicly-traded company, Other: Travel, Accommodations, Expenses. Huang: BeiGene: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months, Other: Travel, Accommodations, Expenses; Protara Therapeutics: Current holder of individual stocks in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Robak: Biogen, Abbvie, Octapharma, Janssen: Honoraria, Other: Advisory board; AstraZeneca, Abbvie, Janssen, Octapharma, Gilead,Oncopeptides AB, Pharmacyclics, Pfizer, GlaxoSmithKline, Biogen: Research Funding; Medical University of Lodz: Current Employment. Hillmen: Pharmacyclics: Honoraria, Research Funding; Roche: Research Funding; Gilead: Research Funding; AstraZeneca: Honoraria; SOBI: Honoraria; BeiGene: Honoraria; AbbVie: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Honoraria, Other: Travel, Accommodations, Expenses, Research Funding.
Zanubrutinib is an investigational agent and has not been approved for TN CLL/SLL without del(17p) in the US

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal